
📈 Limited Opportunity: Invest in Early Lung Cancer Detection
📈 Limited Opportunity: Invest in Early Lung Cancer Detection
📈 Limited Opportunity: Invest in Early Lung Cancer Detection

Detect Early: Save Lives
📈 Limited Opportunity: Invest in Early Lung Cancer Detection

Detect Early: Save Lives



HEAR FROM OUR SCIENTIFIC TEAM

Invest in the Future of Cancer Detection
Invest in the Future of Cancer Detection
Invest in the Future of Cancer Detection
Cizzle Bio is driving a new era in early cancer detection with two breakthrough blood tests—CIZ1B for early-stage lung cancer and DEX-G2 for gastric cancer, two of the deadliest cancers worldwide.
These simple, minimally invasive tests detect cancer early—far earlier than traditional methods—when treatment is more effective and outcomes improve.
Backed by leading U.S. cancer centers, protected by a strong patent portfolio, and preparing for commercial launch in CLIA-certified labs, we are at the threshold of delivering life-saving innovation at scale.
Now is your chance to invest in a company with clinical validation, cutting-edge science, and a clear path to market.
Cizzle Bio is driving a new era in early cancer detection with two breakthrough blood tests—CIZ1B for early-stage lung cancer and DEX-G2 for gastric cancer, two of the deadliest cancers worldwide.
These simple, minimally invasive tests detect cancer early—far earlier than traditional methods—when treatment is more effective and outcomes improve.
Backed by leading U.S. cancer centers, protected by a strong patent portfolio, and preparing for commercial launch in CLIA-certified labs, we are at the threshold of delivering life-saving innovation at scale.
Now is your chance to invest in a company with clinical validation, cutting-edge science, and a clear path to market.
Cizzle Bio is driving a new era in early cancer detection with two breakthrough blood tests—CIZ1B for early-stage lung cancer and DEX-G2 for gastric cancer, two of the deadliest cancers worldwide.
These simple, minimally invasive tests detect cancer early—far earlier than traditional methods—when treatment is more effective and outcomes improve.
Backed by leading U.S. cancer centers, protected by a strong patent portfolio, and preparing for commercial launch in CLIA-certified labs, we are at the threshold of delivering life-saving innovation at scale.
Now is your chance to invest in a company with clinical validation, cutting-edge science, and a clear path to market.
$0.38
$0.38
Share Price
$750
Minimum Investment
$750
Minimum Investment
$750
Minimum
Investment
$163,000+
$163,000+
Total Crowdfunded
$163,000+
Total Crowdfunded
$3.95 M+
$3.95 M+
Raised
$3.95 M+
Raised
FAST FACTS
FAST FACTS
FAST FACTS
FAST FACTS
95% Sensitive
95% Sensitive
95% Sensitive
Our CIZ1B and DEX-G2 Biomarker test shows remarkable accuracy in early clinical studies for detecting early-stage cancer.
Our CIZ1B and DEX-G2 Biomarker test shows remarkable accuracy in early clinical studies for detecting early-stage cancer.
Our CIZ1B and DEX-G2 Biomarker test shows remarkable accuracy in early clinical studies for detecting early-stage cancer.
$95B+ Market
$95B+ Market
$95B+ Market
The global cancer biomarker market estimated to reach $95B by 2032, with a CAGR of 12.9% in the growth period. Source here
Urgent Clinical Need
Urgent Clinical Need
Urgent Clinical Need
Together, lung and gastric cancer claim more than 2.5 million lives each year—most diagnosed too late for effective treatment. We offer early, life-saving detection.
Together, lung and gastric cancer claim more than 2.5 million lives each year—most diagnosed too late for effective treatment. We offer early, life-saving detection.
Together, lung and gastric cancer claim more than 2.5 million lives each year—most diagnosed too late for effective treatment. We offer early, life-saving detection.






Prestigious Research Partnerships
Emerging Site

Washington, DC
Emerging Site

Validating


Durham, NC
Validating
Validating
Los Angeles, CA

Validating
Newport Beach, CA

Validating
San Antonio, TX

Validating
Tampa, FL

Clinical Validation at Top U.S. Cancer Centers
FEATURED IN
FEATURED IN
Learn how Cizzle Bio can save lives through early detection testing.
Learn how Cizzle Bio can save lives
through early detection testing.
Learn how Cizzle Bio can save lives through early detection testing.
Get the Cizzle Bio Investor Deck.
Get the Cizzle Bio Investor Deck.
Get the Cizzle Bio Investor Deck.
PROBLEM
PROBLEM
PROBLEM
PROBLEM
Lung Cancer is the Deadliest Cancer Worldwide
Lung Cancer is the Deadliest
Cancer Worldwide
Lung Cancer is the Deadliest
Cancer Worldwide
Late detection leads to high mortality rates.
Late detection leads to high mortality rates.
Late detection leads to high mortality rates.
Every day, nearly 5,000 lives are lost to late-stage diagnoses. Approximately 75% of lung cancer cases are diagnosed at a late stage, drastically reducing survival rates.
Every day, nearly 5,000 lives are lost to late-stage diagnoses. Approximately 75% of lung cancer cases are diagnosed at a late stage, drastically reducing survival rates.
Every day, nearly 5,000 lives are lost to late-stage diagnoses. Approximately 75% of lung cancer cases are diagnosed at a late stage, drastically reducing survival rates.
The odds are startling: 1 in 16 individuals will be diagnosed with lung cancer at some point in their lives.
Learn more at the Lung Cancer Foundation of America.
The odds are startling: 1 in 16 individuals will be diagnosed with lung cancer at some point in their lives.
Learn more at the Lung Cancer Foundation of America.
The odds are startling: 1 in 16 individuals will be diagnosed with lung cancer at some point in their lives.
Learn more at the Lung Cancer Foundation of America.











SOLUTION
SOLUTION
SOLUTION
SOLUTION
Introducing the CIZ1B Biomarker Test: A Breakthrough in Early Lung Cancer Detection
Introducing the CIZ1B Biomarker Test: A Breakthrough in Early Lung Cancer Detection
Introducing the CIZ1B Biomarker Test: A Breakthrough in Early Lung Cancer Detection
The screening tool patients need and healthcare systems demand. Our CIZ1B Biomarker test offers a simple blood test for early-stage lung cancer detection with 95% sensitivity in early clinical studies.
Low Cost
Highly Accurate
Minimally Invasive
Rapid Results
The screening tool patients need and healthcare systems demand. Our CIZ1B Biomarker test offers a simple blood test for early-stage lung cancer detection with 95% sensitivity in early clinical studies.
Low Cost
Highly Accurate
Minimally Invasive
Rapid Results
The screening tool patients need and healthcare systems demand. Our CIZ1B Biomarker test offers a simple blood test for early-stage lung cancer detection with 95% sensitivity in early clinical studies.
Low Cost
Highly Accurate
Minimally Invasive
Rapid Results
Detecting Early Saves Lives
Detecting Early Saves Lives
Detecting Early Saves Lives
Lung cancer can take many years to be symptomatic when it is at an advanced stage. It is often the case that people who develop lung cancer don't visit a medical care facility for screening until it's too late. Cizzle Bio's technology would allow at risk patients to test preventatively, significantly reducing risk of late detection.
Lung cancer can take many years to be symptomatic when it is at an advanced stage. It is often the case that people who develop lung cancer don't visit a medical care facility for screening until it's too late. Cizzle Bio's technology would allow at risk patients to test preventatively, significantly reducing risk of late detection.
Lung cancer can take many years to be symptomatic when it is at an advanced stage. It is often the case that people who develop lung cancer don't visit a medical care facility for screening until it's too late. Cizzle Bio's technology would allow at risk patients to test preventatively, significantly reducing risk of late detection.


AT RISK



Symptoms



Imaging



Biopsy



Treatment


Recurrence






MARKET
MARKET
MARKET
MARKET
Capturing a $95B+ Opportunity in
Rapidly Growing Diagnostics Market
Capturing a $95B+ Opportunity in
Rapidly Growing Diagnostics Market
Capturing a $95B+ Opportunity in
Rapidly Growing Diagnostics Market
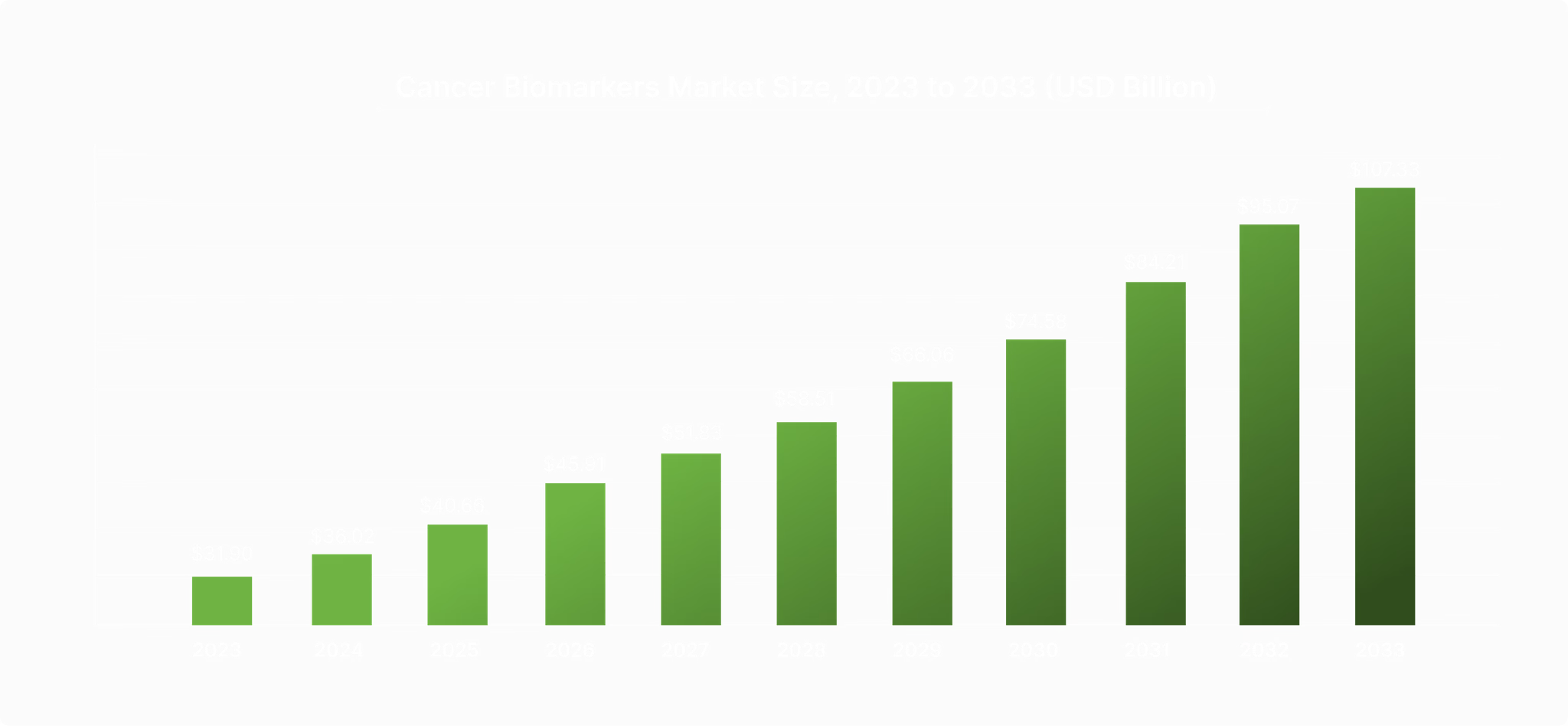
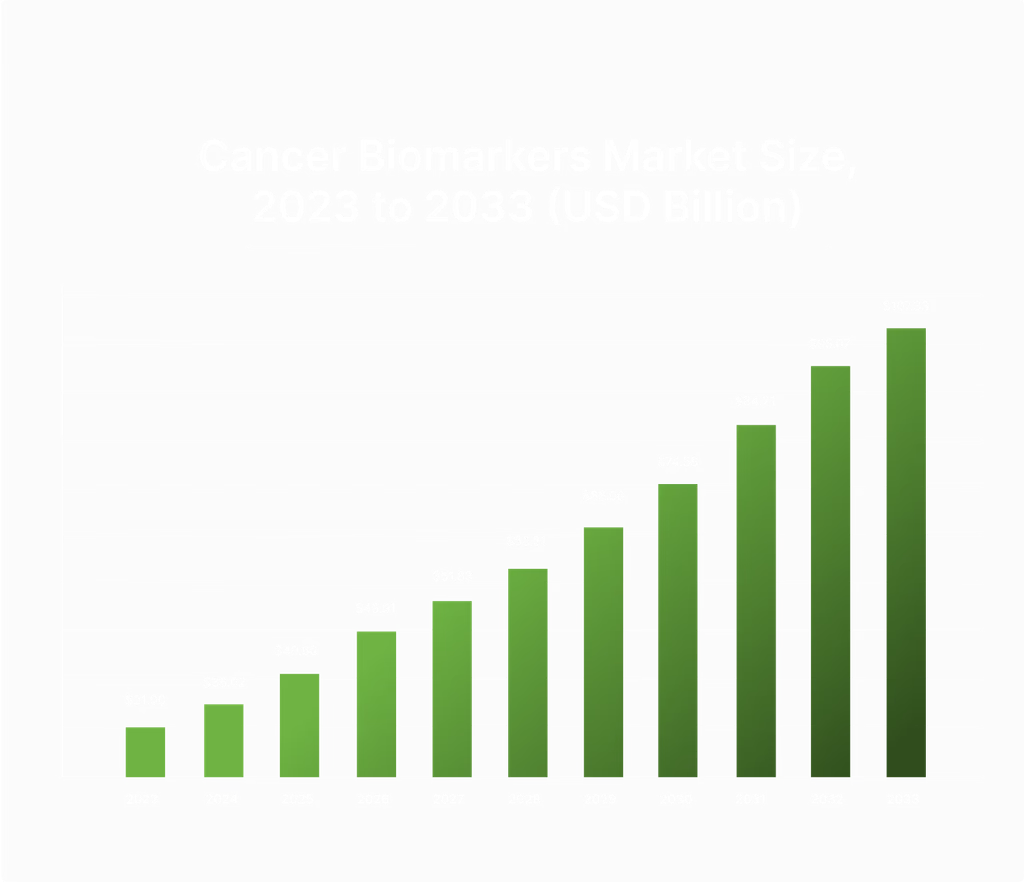
The cancer biomarkers market is set to grow from $31.90 billion in 2023 to $107.33 billion by 2033, fueled by the rising demand for early detection and precision diagnostics. Cizzle is uniquely positioned to tap into this $95B+ market with its focus on advancing early lung cancer detection through biomarker technology.
The cancer biomarkers market is set to grow from $31.90 billion in 2023 to $107.33 billion by 2033, fueled by the rising demand for early detection and precision diagnostics. Cizzle is uniquely positioned to tap into this $95B+ market with its focus on advancing early lung cancer detection through biomarker technology.
The cancer biomarkers market is set to grow from $31.90 billion in 2023 to $107.33 billion by 2033, fueled by the rising demand for early detection and precision diagnostics. Cizzle is uniquely positioned to tap into this $95B+ market with its focus on advancing early lung cancer detection through biomarker technology.








BUSINESS MODEL
BUSINESS MODEL
BUSINESS MODEL
BUSINESS MODEL
Advancing Lung Cancer Diagnostics with CIZ1B to $33.8M/Yr Revenue by Year Three*
Advancing Lung Cancer Diagnostics with CIZ1B to $33.8M/Yr Revenue by Year Three*
Advancing Lung Cancer Diagnostics with CIZ1B to $33.8M/Yr Revenue by Year Three*
Cizzle Bio's business model is powered by our proprietary CIZ1B blood test for early-stage lung cancer detection, generating revenue through initial test deployment, clinical diagnostics usage, and high-value royalties.
Based on our projections, we are poised to achieve:*
$10K to $1.4M revenue growth from lab tests in Year 1
$340K to $1.2M POC test revenue in Year 1
Recurring revenue reaching $4.6M by Year 3
2,033% YoY revenue growth from Year 2 to Year 3
Net royalty scaling to $4.6M by final month of Year 3
*Future projections are not guaranteed results.
Cizzle Bio's business model is powered by our proprietary CIZ1B blood test for early-stage lung cancer detection, generating revenue through initial test deployment, clinical diagnostics usage, and high-value royalties.
Based on our projections, we are poised to achieve:*
$10K to $1.4M revenue growth from lab tests in Year 1
$340K to $1.2M POC test revenue in Year 1
Recurring revenue reaching $4.6M by Year 3
2,033% YoY revenue growth from Year 2 to Year 3
Net royalty scaling to $4.6M by final month of Year 3
*Future projections are not guaranteed results.
Cizzle Bio's business model is powered by our proprietary CIZ1B blood test for early-stage lung cancer detection, generating revenue through initial test deployment, clinical diagnostics usage, and high-value royalties.
Based on our projections, we are poised to achieve:*
$10K to $1.4M revenue growth from lab tests in Year 1
$340K to $1.2M POC test revenue in Year 1
Recurring revenue reaching $4.6M by Year 3
2,033% YoY revenue growth from Year 2 to Year 3
Net royalty scaling to $4.6M by final month of Year 3
*Future projections are not guaranteed results.

COMPETITION
COMPETITION
COMPETITION
COMPETITION
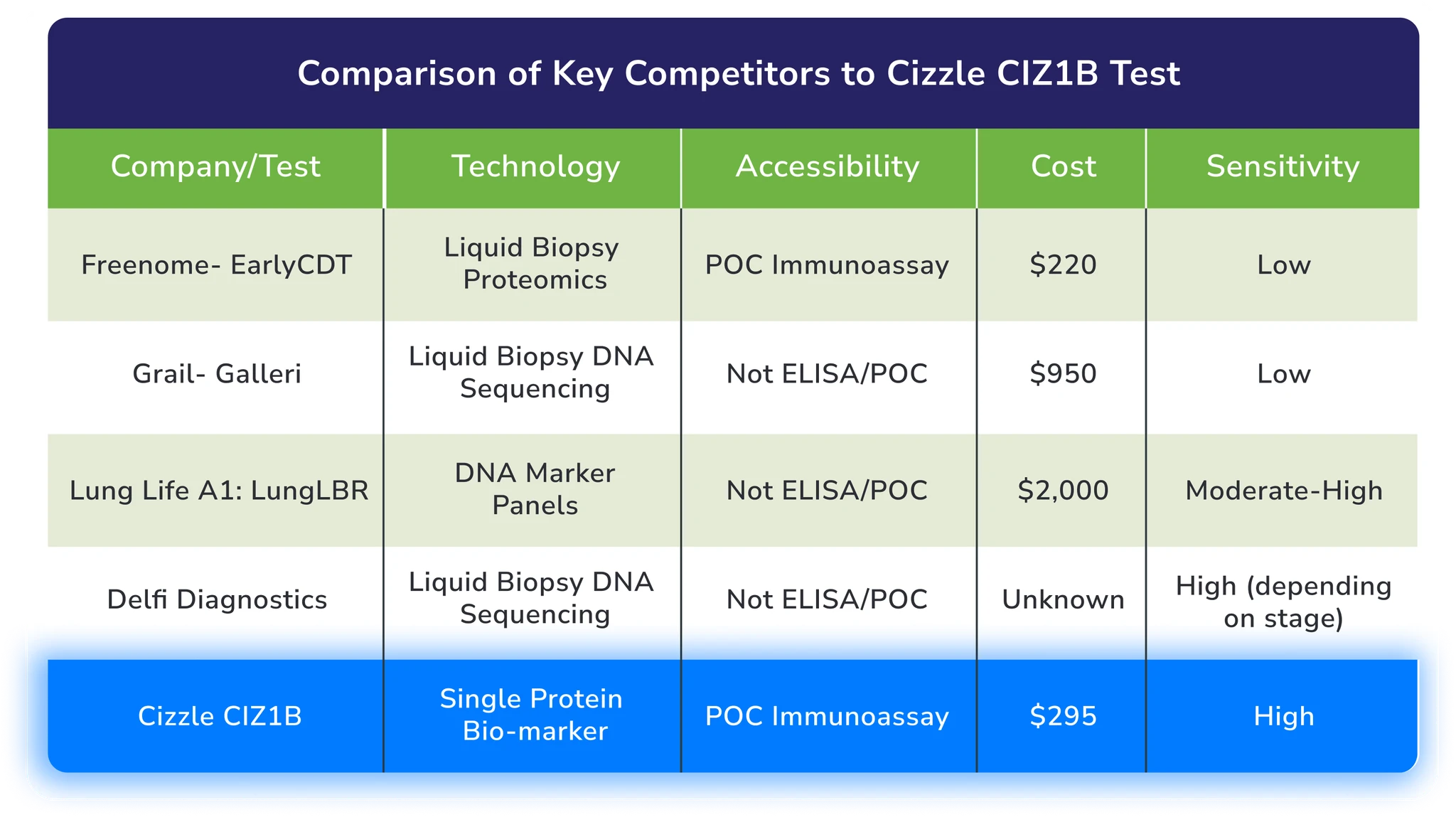
Unrivaled in Accuracy, Simplicity, and Cost-Effectiveness
Unrivaled in Accuracy, Simplicity, and Cost-Effectiveness
Unrivaled in Accuracy, Simplicity, and Cost-Effectiveness
While competitors struggle with invasive procedures and high false-positive rates, Cizzle Bio's CIZ1B Biomarker test offers unparalleled capabilities. Our test delivers early detection with 95% sensitivity – significantly more accurate than current methods, with exceptional ease-of-use and cost-effectiveness.
While competitors struggle with invasive procedures and high false-positive rates, Cizzle Bio's CIZ1B Biomarker test offers unparalleled capabilities. Our test delivers early detection with 95% sensitivity – significantly more accurate than current methods, with exceptional ease-of-use and cost-effectiveness.
While competitors struggle with invasive procedures and high false-positive rates, Cizzle Bio's CIZ1B Biomarker test offers unparalleled capabilities. Our test delivers early detection with 95% sensitivity – significantly more accurate than current methods, with exceptional ease-of-use and cost-effectiveness.



TEAM
TEAM
TEAM
TEAM
Biotech and Healthcare Experts
Leading the Charge
Biotech and Healthcare Experts
Leading the Charge
Biotech and Healthcare Experts Leading the Charge



Bill Behnke
Bill Behnke
Bill Behnke
CHAIRMAN & CEO
CHAIRMAN & CEO
CHAIRMAN & CEO
Has raised over $100M in capital and is an expert in business development. Worked with major cancer charities and health insurers, including the Leukemia and Lymphoma Society.
Has raised over $100M in capital and is an expert in business development. Worked with major cancer charities and health insurers, including the Leukemia and Lymphoma Society.
Has raised over $100M in capital and is an expert in business development. Worked with major cancer charities and health insurers, including the Leukemia and Lymphoma Society.



Dr. Ron Greeno
Dr. Ron Greeno
Dr. Ron Greeno
MD, FCCP, MHM
NON-EXECUTIVE DIRECTOR
MD, FCCP, MHM
NON-EXECUTIVE DIRECTOR
MD, FCCP, MHM
NON-EXECUTIVE DIRECTOR
Founder of Cogent Healthcare, and a recognized leader in physician services. Served as a strategic healthcare policy advisor on Capitol Hill, Medicare, and CMMI.
Founder of Cogent Healthcare, and a recognized leader in physician services. Served as a strategic healthcare policy advisor on Capitol Hill, Medicare, and CMMI.
Founder of Cogent Healthcare, and a recognized leader in physician services. Served as a strategic healthcare policy advisor on Capitol Hill, Medicare, and CMMI.



Mary Jimenez
Mary Jimenez
Mary Jimenez
EXECUTIVE VICE PRESIDENT,
BUSINESS DEVELOPMENT
EXECUTIVE VICE PRESIDENT,
BUSINESS DEVELOPMENT
EXECUTIVE VICE PRESIDENT,
BUSINESS DEVELOPMENT
Former executive at Genentech and Caris, specializing in biotech sales. Has led successful business strategies and sales teams in the biotechnology sector.
Former executive at Genentech and Caris, specializing in biotech sales. Has led successful business strategies and sales teams in the biotechnology sector.
Former executive at Genentech and Caris, specializing in biotech sales. Has led successful business strategies and sales teams in the biotechnology sector.



Dr. Allan Syms
Dr. Allan Syms
Dr. Allan Syms
Executive Chairman
Executive Chairman
Executive Chairman
Over 30 years of experience in biotech, leading companies from a to IPO. Former Corporate Marketing Director at Integra Biosciences AG.
Over 30 years of experience in biotech, leading companies from a to IPO. Former Corporate Marketing Director at Integra Biosciences AG.
Over 30 years of experience in biotech, leading companies from a to IPO. Former Corporate Marketing Director at Integra Biosciences AG.



Nigel Lee
Nigel Lee
Nigel Lee
FCA
FCA
FCA
Director of CFO Solutions Ltd., providing financial advisory services. Held senior management roles at PricewaterhouseCoopers, overseeing financial operations for biotech initiatives.
Director of CFO Solutions Ltd., providing financial advisory services. Held senior management roles at PricewaterhouseCoopers, overseeing financial operations for biotech initiatives.
Director of CFO Solutions Ltd., providing financial advisory services. Held senior management roles at PricewaterhouseCoopers, overseeing financial operations for biotech initiatives.



Prof. Dawn Coverely
Prof. Dawn Coverely
Prof. Dawn Coverely
FOUNDER
FOUNDER
FOUNDER
Cancer biologist and founder of Cizzle Biotechnology. Known for significant contributions to oncology research and the development of cancer screening technologies.
Cancer biologist and founder of Cizzle Biotechnology. Known for significant contributions to oncology research and the development of cancer screening technologies.
Cancer biologist and founder of Cizzle Biotechnology. Known for significant contributions to oncology research and the development of cancer screening technologies.
GO TO MARKET
GO TO MARKET
GO TO MARKET
GO TO MARKET
Streamlined Product Roadmap to Revenue
Streamlined Product Roadmap to Revenue
Streamlined Product Roadmap to Revenue
Clinical and Regulatory
Clinical and Regulatory
Clinical and Regulatory
Objective: Complete additional clinical trials where necessary, complete CLIA (Clinical Laboratory Improvement Amendments) certification for LDTs (Laboratory Developed Tests), and secure insurance reimbursement pathways.
Objective: Complete additional clinical trials where necessary, complete CLIA (Clinical Laboratory Improvement Amendments) certification for LDTs (Laboratory Developed Tests), and secure insurance reimbursement pathways.
Objective: Complete additional clinical trials where necessary, complete CLIA (Clinical Laboratory Improvement Amendments) certification for LDTs (Laboratory Developed Tests), and secure insurance reimbursement pathways.
1
1
1
Launch and Early
Adoption
Launch and Early
Adoption
Launch and Early
Adoption
Objective: Begin scaling manufacturing and commercialization efforts. Focus on partnerships with hospitals and research institutions for early adoption.
Objective: Begin scaling manufacturing and commercialization efforts. Focus on partnerships with hospitals and research institutions for early adoption.
Objective: Begin scaling manufacturing and commercialization efforts. Focus on partnerships with hospitals and research institutions for early adoption.
2
2
2
Scaling and Market Capture
Scaling and Market Capture
Scaling and Market Capture
Objective: Achieve target SOM market penetration in the U.S., supported by strong data and partnerships. Expand diagnostic portfolio to include early detection tests for pancreatic, colon, esophageal cancers, and Alzheimer’s.
Objective: Achieve target SOM market penetration in the U.S., supported by strong data and partnerships. Expand diagnostic portfolio to include early detection tests for pancreatic, colon, esophageal cancers, and Alzheimer’s.
Objective: Achieve target SOM market penetration in the U.S., supported by strong data and partnerships. Expand diagnostic portfolio to include early detection tests for pancreatic, colon, esophageal cancers, and Alzheimer’s.
3
3
3


FAQ
FAQ
FAQ
HOW MUCH CAN I INVEST?
HOW MUCH CAN I INVEST?
HOW MUCH CAN I INVEST?
HOW MUCH CAN I INVEST?
HOW DO I CALCULATE MY NET WORTH?
HOW DO I CALCULATE MY NET WORTH?
HOW DO I CALCULATE MY NET WORTH?
HOW DO I CALCULATE MY NET WORTH?
WHAT ARE THE TAX IMPLICATIONS OF AN EQUITY CROWDFUNDING INVESTMENT?
WHAT ARE THE TAX IMPLICATIONS OF AN EQUITY CROWDFUNDING INVESTMENT?
WHAT ARE THE TAX IMPLICATIONS OF AN EQUITY CROWDFUNDING INVESTMENT?
WHAT ARE THE TAX IMPLICATIONS OF AN EQUITY CROWDFUNDING INVESTMENT?
WHO CAN INVEST IN A REGULATION CF OFFERING?
WHO CAN INVEST IN A REGULATION CF OFFERING?
WHO CAN INVEST IN A REGULATION CF OFFERING?
WHO CAN INVEST IN A REGULATION CF OFFERING?
WHAT DO I NEED TO KNOW ABOUT EARLY-STAGE INVESTING? ARE THESE INVESTMENTS RISKY?
WHAT DO I NEED TO KNOW ABOUT EARLY-STAGE INVESTING? ARE THESE INVESTMENTS RISKY?
WHAT DO I NEED TO KNOW ABOUT EARLY-STAGE INVESTING? ARE THESE INVESTMENTS RISKY?
WHAT DO I NEED TO KNOW ABOUT EARLY-STAGE INVESTING? ARE THESE INVESTMENTS RISKY?
CAN I SELL MY UNITS?
CAN I SELL MY UNITS?
CAN I SELL MY UNITS?
CAN I SELL MY UNITS?
WHAT HAPPENS IF A COMPANY DOES NOT REACH THEIR FUNDING TARGET?
WHAT HAPPENS IF A COMPANY DOES NOT REACH THEIR FUNDING TARGET?
WHAT HAPPENS IF A COMPANY DOES NOT REACH THEIR FUNDING TARGET?
WHAT HAPPENS IF A COMPANY DOES NOT REACH THEIR FUNDING TARGET?
HOW CAN I LEARN MORE ABOUT A COMPANY’S OFFERING?
HOW CAN I LEARN MORE ABOUT A COMPANY’S OFFERING?
HOW CAN I LEARN MORE ABOUT A COMPANY’S OFFERING?
HOW CAN I LEARN MORE ABOUT A COMPANY’S OFFERING?
WHAT IF I CHANGE MY MIND ABOUT INVESTING?
WHAT IF I CHANGE MY MIND ABOUT INVESTING?
WHAT IF I CHANGE MY MIND ABOUT INVESTING?
WHAT IF I CHANGE MY MIND ABOUT INVESTING?
HOW CAN I KEEP UP WITH HOW THE COMPANY IS DOING?
HOW CAN I KEEP UP WITH HOW THE COMPANY IS DOING?
HOW CAN I KEEP UP WITH HOW THE COMPANY IS DOING?
HOW CAN I KEEP UP WITH HOW THE COMPANY IS DOING?
WHAT RELATIONSHIP DOES THE COMPANY HAVE WITH DEALMAKER SECURITIES?
WHAT RELATIONSHIP DOES THE COMPANY HAVE WITH DEALMAKER SECURITIES?
WHAT RELATIONSHIP DOES THE COMPANY HAVE WITH DEALMAKER SECURITIES?
WHAT RELATIONSHIP DOES THE COMPANY HAVE WITH DEALMAKER SECURITIES?
WHAT IS THE MINIMUM INVESTMENT AMOUNT?
WHAT IS THE MINIMUM INVESTMENT AMOUNT?
WHAT IS THE MINIMUM INVESTMENT AMOUNT?
WHAT IS THE MINIMUM INVESTMENT AMOUNT?
DISCLAIMER: Equity crowdfunding investments in private placements, and start-up investments in particular, are speculative and involve a high degree of risk and those investors who cannot afford to lose their entire investment should not invest in start-ups. Companies seeking startup investment through equity crowdfunding tend to be in earlier stages of development and their business model, products and services may not yet be fully developed, operational or tested in the public marketplace. There is no guarantee that the stated valuation and other terms are accurate or in agreement with the market or industry valuations. Further, investors may receive illiquid and/or restricted stock that may be subject to holding period requirements and/or liquidity concerns.
DealMaker Securities LLC, a registered broker-dealer, and member of FINRA | SIPC, located at 4000 Eagle Point Corporate Drive, Suite 950, Birmingham, AL 35242., is the Intermediary for this offering and is not an affiliate of or connected with the Issuer. Please check our background on FINRA's BrokerCheck.
DealMaker Securities LLC does not make investment recommendations.
DealMaker Securities LLC is NOT placing or selling these securities on behalf of the Issuer.
DealMaker Securities LLC is NOT soliciting this investment or making any recommendations by collecting, reviewing, and processing an Investor's documentation for this investment.
DealMaker Securities LLC conducts Anti-Money Laundering, Identity and Bad Actor Disqualification reviews of the Issuer, and confirms they are a registered business in good standing.
DealMaker Securities LLC is NOT vetting or approving the information provided by the Issuer or the Issuer itself.
Contact information is provided for Investors to make inquiries and requests to DealMaker Securities LLC regarding Regulation CF in general, or the status of such investor’s submitted documentation, specifically. DealMaker Securities LLC may direct Investors to specific sections of the Offering Circular to locate information or answers to their inquiry but does not opine or provide guidance on issuer related matters.
THIS WEBSITE MAY CONTAIN FORWARD-LOOKING STATEMENTS AND INFORMATION RELATING TO, AMONG OTHER THINGS, THE COMPANY, ITS BUSINESS PLAN AND STRATEGY, AND ITS INDUSTRY. THESE FORWARD-LOOKING STATEMENTS ARE BASED ON THE BELIEFS OF, ASSUMPTIONS MADE BY, AND INFORMATION CURRENTLY AVAILABLE TO THE COMPANY'S MANAGEMENT. WHEN USED IN THE OFFERING MATERIALS, THE WORDS "ESTIMATE," "PROJECT," "BELIEVE," "ANTICIPATE," "INTEND," "EXPECT" AND SIMILAR EXPRESSIONS ARE INTENDED TO IDENTIFY FORWARD-LOOKING STATEMENTS. THESE STATEMENTS REFLECT MANAGEMENT'S CURRENT VIEWS WITH RESPECT TO FUTURE EVENTS AND ARE SUBJECT TO RISKS AND UNCERTAINTIES THAT COULD CAUSE THE COMPANY'S ACTUAL RESULTS TO DIFFER MATERIALLY FROM THOSE CONTAINED IN THE FORWARD-LOOKING STATEMENTS. INVESTORS ARE CAUTIONED NOT TO PLACE UNDUE RELIANCE ON THESE FORWARD-LOOKING STATEMENTS, WHICH SPEAK ONLY AS OF THE DATE ON WHICH THEY ARE MADE. THE COMPANY DOES NOT UNDERTAKE ANY OBLIGATION TO REVISE OR UPDATE THESE FORWARD-LOOKING STATEMENTS TO REFLECT EVENTS OR CIRCUMSTANCES AFTER SUCH DATE OR TO REFLECT THE OCCURRENCE OF UNANTICIPATED EVENTS.
DISCLAIMER: Equity crowdfunding investments in private placements, and start-up investments in particular, are speculative and involve a high degree of risk and those investors who cannot afford to lose their entire investment should not invest in start-ups. Companies seeking startup investment through equity crowdfunding tend to be in earlier stages of development and their business model, products and services may not yet be fully developed, operational or tested in the public marketplace. There is no guarantee that the stated valuation and other terms are accurate or in agreement with the market or industry valuations. Further, investors may receive illiquid and/or restricted stock that may be subject to holding period requirements and/or liquidity concerns.
DealMaker Securities LLC, a registered broker-dealer, and member of FINRA | SIPC, located at 4000 Eagle Point Corporate Drive, Suite 950, Birmingham, AL 35242., is the Intermediary for this offering and is not an affiliate of or connected with the Issuer. Please check our background on FINRA's BrokerCheck.
DealMaker Securities LLC does not make investment recommendations.
DealMaker Securities LLC is NOT placing or selling these securities on behalf of the Issuer.
DealMaker Securities LLC is NOT soliciting this investment or making any recommendations by collecting, reviewing, and processing an Investor's documentation for this investment.
DealMaker Securities LLC conducts Anti-Money Laundering, Identity and Bad Actor Disqualification reviews of the Issuer, and confirms they are a registered business in good standing.
DealMaker Securities LLC is NOT vetting or approving the information provided by the Issuer or the Issuer itself.
Contact information is provided for Investors to make inquiries and requests to DealMaker Securities LLC regarding Regulation CF in general, or the status of such investor’s submitted documentation, specifically. DealMaker Securities LLC may direct Investors to specific sections of the Offering Circular to locate information or answers to their inquiry but does not opine or provide guidance on issuer related matters.
THIS WEBSITE MAY CONTAIN FORWARD-LOOKING STATEMENTS AND INFORMATION RELATING TO, AMONG OTHER THINGS, THE COMPANY, ITS BUSINESS PLAN AND STRATEGY, AND ITS INDUSTRY. THESE FORWARD-LOOKING STATEMENTS ARE BASED ON THE BELIEFS OF, ASSUMPTIONS MADE BY, AND INFORMATION CURRENTLY AVAILABLE TO THE COMPANY'S MANAGEMENT. WHEN USED IN THE OFFERING MATERIALS, THE WORDS "ESTIMATE," "PROJECT," "BELIEVE," "ANTICIPATE," "INTEND," "EXPECT" AND SIMILAR EXPRESSIONS ARE INTENDED TO IDENTIFY FORWARD-LOOKING STATEMENTS. THESE STATEMENTS REFLECT MANAGEMENT'S CURRENT VIEWS WITH RESPECT TO FUTURE EVENTS AND ARE SUBJECT TO RISKS AND UNCERTAINTIES THAT COULD CAUSE THE COMPANY'S ACTUAL RESULTS TO DIFFER MATERIALLY FROM THOSE CONTAINED IN THE FORWARD-LOOKING STATEMENTS. INVESTORS ARE CAUTIONED NOT TO PLACE UNDUE RELIANCE ON THESE FORWARD-LOOKING STATEMENTS, WHICH SPEAK ONLY AS OF THE DATE ON WHICH THEY ARE MADE. THE COMPANY DOES NOT UNDERTAKE ANY OBLIGATION TO REVISE OR UPDATE THESE FORWARD-LOOKING STATEMENTS TO REFLECT EVENTS OR CIRCUMSTANCES AFTER SUCH DATE OR TO REFLECT THE OCCURRENCE OF UNANTICIPATED EVENTS.
DISCLAIMER: Equity crowdfunding investments in private placements, and start-up investments in particular, are speculative and involve a high degree of risk and those investors who cannot afford to lose their entire investment should not invest in start-ups. Companies seeking startup investment through equity crowdfunding tend to be in earlier stages of development and their business model, products and services may not yet be fully developed, operational or tested in the public marketplace. There is no guarantee that the stated valuation and other terms are accurate or in agreement with the market or industry valuations. Further, investors may receive illiquid and/or restricted stock that may be subject to holding period requirements and/or liquidity concerns.
DealMaker Securities LLC, a registered broker-dealer, and member of FINRA | SIPC, located at 4000 Eagle Point Corporate Drive, Suite 950, Birmingham, AL 35242., is the Intermediary for this offering and is not an affiliate of or connected with the Issuer. Please check our background on FINRA's BrokerCheck.
DealMaker Securities LLC does not make investment recommendations.
DealMaker Securities LLC is NOT placing or selling these securities on behalf of the Issuer.
DealMaker Securities LLC is NOT soliciting this investment or making any recommendations by collecting, reviewing, and processing an Investor's documentation for this investment.
DealMaker Securities LLC conducts Anti-Money Laundering, Identity and Bad Actor Disqualification reviews of the Issuer, and confirms they are a registered business in good standing.
DealMaker Securities LLC is NOT vetting or approving the information provided by the Issuer or the Issuer itself.
Contact information is provided for Investors to make inquiries and requests to DealMaker Securities LLC regarding Regulation CF in general, or the status of such investor’s submitted documentation, specifically. DealMaker Securities LLC may direct Investors to specific sections of the Offering Circular to locate information or answers to their inquiry but does not opine or provide guidance on issuer related matters.
THIS WEBSITE MAY CONTAIN FORWARD-LOOKING STATEMENTS AND INFORMATION RELATING TO, AMONG OTHER THINGS, THE COMPANY, ITS BUSINESS PLAN AND STRATEGY, AND ITS INDUSTRY. THESE FORWARD-LOOKING STATEMENTS ARE BASED ON THE BELIEFS OF, ASSUMPTIONS MADE BY, AND INFORMATION CURRENTLY AVAILABLE TO THE COMPANY'S MANAGEMENT. WHEN USED IN THE OFFERING MATERIALS, THE WORDS "ESTIMATE," "PROJECT," "BELIEVE," "ANTICIPATE," "INTEND," "EXPECT" AND SIMILAR EXPRESSIONS ARE INTENDED TO IDENTIFY FORWARD-LOOKING STATEMENTS. THESE STATEMENTS REFLECT MANAGEMENT'S CURRENT VIEWS WITH RESPECT TO FUTURE EVENTS AND ARE SUBJECT TO RISKS AND UNCERTAINTIES THAT COULD CAUSE THE COMPANY'S ACTUAL RESULTS TO DIFFER MATERIALLY FROM THOSE CONTAINED IN THE FORWARD-LOOKING STATEMENTS. INVESTORS ARE CAUTIONED NOT TO PLACE UNDUE RELIANCE ON THESE FORWARD-LOOKING STATEMENTS, WHICH SPEAK ONLY AS OF THE DATE ON WHICH THEY ARE MADE. THE COMPANY DOES NOT UNDERTAKE ANY OBLIGATION TO REVISE OR UPDATE THESE FORWARD-LOOKING STATEMENTS TO REFLECT EVENTS OR CIRCUMSTANCES AFTER SUCH DATE OR TO REFLECT THE OCCURRENCE OF UNANTICIPATED EVENTS.
DISCLAIMER: Equity crowdfunding investments in private placements, and start-up investments in particular, are speculative and involve a high degree of risk and those investors who cannot afford to lose their entire investment should not invest in start-ups. Companies seeking startup investment through equity crowdfunding tend to be in earlier stages of development and their business model, products and services may not yet be fully developed, operational or tested in the public marketplace. There is no guarantee that the stated valuation and other terms are accurate or in agreement with the market or industry valuations. Further, investors may receive illiquid and/or restricted stock that may be subject to holding period requirements and/or liquidity concerns.
DealMaker Securities LLC, a registered broker-dealer, and member of FINRA | SIPC, located at 4000 Eagle Point Corporate Drive, Suite 950, Birmingham, AL 35242., is the Intermediary for this offering and is not an affiliate of or connected with the Issuer. Please check our background on FINRA's BrokerCheck.
DealMaker Securities LLC does not make investment recommendations.
DealMaker Securities LLC is NOT placing or selling these securities on behalf of the Issuer.
DealMaker Securities LLC is NOT soliciting this investment or making any recommendations by collecting, reviewing, and processing an Investor's documentation for this investment.
DealMaker Securities LLC conducts Anti-Money Laundering, Identity and Bad Actor Disqualification reviews of the Issuer, and confirms they are a registered business in good standing.
DealMaker Securities LLC is NOT vetting or approving the information provided by the Issuer or the Issuer itself.
Contact information is provided for Investors to make inquiries and requests to DealMaker Securities LLC regarding Regulation CF in general, or the status of such investor’s submitted documentation, specifically. DealMaker Securities LLC may direct Investors to specific sections of the Offering Circular to locate information or answers to their inquiry but does not opine or provide guidance on issuer related matters.
THIS WEBSITE MAY CONTAIN FORWARD-LOOKING STATEMENTS AND INFORMATION RELATING TO, AMONG OTHER THINGS, THE COMPANY, ITS BUSINESS PLAN AND STRATEGY, AND ITS INDUSTRY. THESE FORWARD-LOOKING STATEMENTS ARE BASED ON THE BELIEFS OF, ASSUMPTIONS MADE BY, AND INFORMATION CURRENTLY AVAILABLE TO THE COMPANY'S MANAGEMENT. WHEN USED IN THE OFFERING MATERIALS, THE WORDS "ESTIMATE," "PROJECT," "BELIEVE," "ANTICIPATE," "INTEND," "EXPECT" AND SIMILAR EXPRESSIONS ARE INTENDED TO IDENTIFY FORWARD-LOOKING STATEMENTS. THESE STATEMENTS REFLECT MANAGEMENT'S CURRENT VIEWS WITH RESPECT TO FUTURE EVENTS AND ARE SUBJECT TO RISKS AND UNCERTAINTIES THAT COULD CAUSE THE COMPANY'S ACTUAL RESULTS TO DIFFER MATERIALLY FROM THOSE CONTAINED IN THE FORWARD-LOOKING STATEMENTS. INVESTORS ARE CAUTIONED NOT TO PLACE UNDUE RELIANCE ON THESE FORWARD-LOOKING STATEMENTS, WHICH SPEAK ONLY AS OF THE DATE ON WHICH THEY ARE MADE. THE COMPANY DOES NOT UNDERTAKE ANY OBLIGATION TO REVISE OR UPDATE THESE FORWARD-LOOKING STATEMENTS TO REFLECT EVENTS OR CIRCUMSTANCES AFTER SUCH DATE OR TO REFLECT THE OCCURRENCE OF UNANTICIPATED EVENTS.










